Can A Change In Pressure Influence Equilibrium In All Reversible Reactions?
Gcse Chemistry – Reversible Reactions And Equilibrium #49
Keywords searched by users: Can a change in pressure shift the equilibrium position in every reversible reaction explain how can changes in temperature shift the position of equilibrium?, for a chemical equilibrium to be established, the chemical reaction must be irreversible., what change can result in a shift in equilibrium during a reaction?, how can you use a balanced chemical equation to write an equilibrium constant expression, in an exothermic reaction, equilibrium shifts _____ when temperature is raised., keq ___ 1: reactants are favored at equilibrium., Equilibrium shift, Le Chatelier’s Principle explained
Can A Pressure Change Shift The Direction Of Equilibrium In Every Reversible Reaction?
Can a pressure change influence the equilibrium position in all reversible reactions? The impact of a pressure change on equilibrium varies depending on the specific reaction. In some cases, altering the pressure can indeed shift the equilibrium, while in others, it may have minimal or no effect. Therefore, it’s essential to consider the nature of the reaction and the reaction conditions when assessing the role of pressure changes in altering equilibrium positions.
How Will Increasing Pressure Affect The Equilibrium Position In A Reversible Reaction?
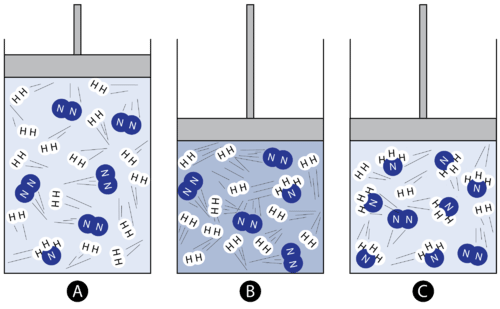
How does altering pressure impact the equilibrium position in a reversible chemical reaction? In reversible reactions, changes in pressure can influence the distribution of reactants and products. Specifically, when pressure is increased, the equilibrium position tends to shift towards the side of the reaction with fewer moles of gas, while a decrease in pressure leads to a shift towards the side with more moles of gas. This dynamic relationship between pressure and equilibrium is a fundamental concept in chemistry and plays a crucial role in understanding reaction behavior. (Date: January 29, 2023)
Share 34 Can a change in pressure shift the equilibrium position in every reversible reaction explain






Categories: Update 33 Can A Change In Pressure Shift The Equilibrium Position In Every Reversible Reaction Explain
See more here: maucongbietthu.com

Can a pressure change shift the equilibrium position in every reversible reaction? Explain. No, because it must be a gas and have an unequal number of moles. Using the following equilibrium constants, determine which reactions would favor the products.No, a pressure change cannot shift the position of equilibrium in all reversible reactions.When there is an increase in pressure, the equilibrium will shift towards the side of the reaction with fewer moles of gas. When there is a decrease in pressure, the equilibrium will shift towards the side of the reaction with more moles of gas.
Learn more about the topic Can a change in pressure shift the equilibrium position in every reversible reaction explain.
- Chapter 18 Flashcards – Quizlet
- Can a pressure change shift the equilibrium position in every …
- Effect of Pressure on Gas-Phase Equilibria – Chemistry LibreTexts
- Shifting Equilibria: Le Chatelier’s Principle – Introductory Chemistry
- Changing the equilibrium position – Higher – Factors affecting the … – BBC
- What happens to equilibrium when pressure is increased? – BYJU’S