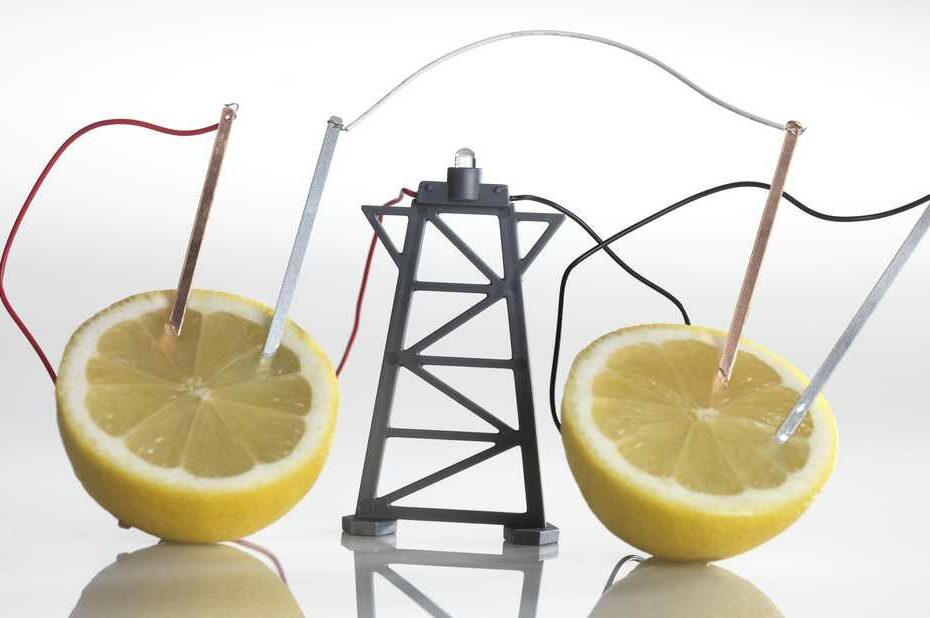
Can A Fruit Battery Power Your Devices?
How To Make A Lemon Battery
Keywords searched by users: Can a fruit battery can perform work why does a potato battery work, how to make a fruit battery, fruit battery experiment, fruit battery experiment pdf, fruit battery experiment results, how to make a citrus battery, make a battery out of fruits and vegetables research paper, what fruit conducts the most electricity
Which Fruit Works Best As A Battery?
Which fruit is most suitable for use as a battery? When it comes to creating a homemade battery from fruit, the choice of fruit is crucial for optimal performance. Citrus fruits like lemons, limes, oranges, and grapefruits are popular choices due to their high citric acid content, which serves as the electrolyte necessary for the battery’s operation. However, it’s important to note that the effectiveness of these fruits can vary, with sweeter, riper ones being less efficient for this purpose. While some experimental reports suggest that apples and pears can be used for similar purposes, they may not perform as well as their citrus counterparts. This information was last reported on July 19, 2019.
Can Fruit Conduct Electricity?
Can fruits and vegetables have the ability to conduct electricity? The answer is yes, and this phenomenon is primarily attributed to the presence of water, which contains extra ions, within these organic materials. In order to harness this electrical conductivity, a simple experiment can be conducted by inserting two metal electrodes into the fruit or vegetable. This setup allows for the generation of electricity, showcasing how these natural components can serve as conductors. This fascinating discovery sheds light on the unique electrical properties inherent in fruits and vegetables, highlighting their potential applications in various scientific experiments and educational demonstrations.
How It Was Possible For The Fruit Battery To Generate Electricity?
The generation of electricity in a fruit battery is made possible through a fascinating chemical reaction involving the lemon’s citric acid, zinc, and copper. When the lemon’s citric acid comes into contact with the zinc, it initiates a reaction that liberates electrons from the zinc electrode. In this process, copper plays a pivotal role because it has a higher affinity for electrons compared to zinc. Consequently, the loose electrons are drawn towards the copper electrode when the two electrodes are connected using wires. This movement of electrons, known as an electric current, is the driving force behind the illumination of the connected light bulb, making it glow and demonstrating the conversion of chemical energy from the lemon into electrical energy that powers the bulb.
Collect 41 Can a fruit battery can perform work
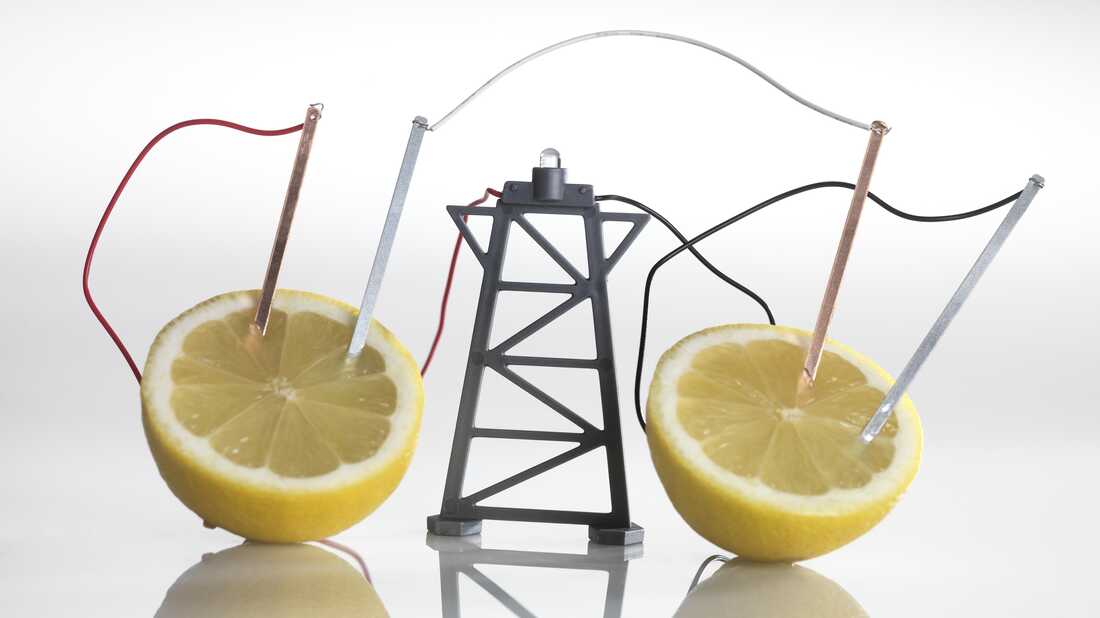






Categories: Discover 59 Can A Fruit Battery Can Perform Work
See more here: maucongbietthu.com
A fruit battery works because some fruits contain citric acid. This acid participates in chemical reactions that make electrons flow, thus creating electricity. However, these fruit batteries have very little voltage, probably less than one volt.Any citrus fruit such as lemons, limes, oranges and grapefruit will work because they all contain citric acid for the electrolyte. However sweet, ripe ones are less effective. We have also heard of experiments using apples and pears although these apparently don’t work as well.Most fruits and vegetables will conduct electricity because they contain water (with extra ions). To generate electricity you need to stick two metal electrodes into the fruit.
Learn more about the topic Can a fruit battery can perform work.
- Fruit Batteries: Do They Work? – California State Science Fair
- Making Fruit Batteries Which One Works Best?
- What makes fruits conduct electricity? – Physics Stack Exchange
- Lemon Battery – Science World
- Lemon Battery – Panasonic Energy Co., Ltd.
- Fruit Batteries: Do They Work? – California State Science Fair